bond order formula
Want to ace chemistry. In bonding moleclar orbitals nb is the number of electrons.
 |
| 3 Ways To Calculate Bond Order In Chemistry Wikihow |
Bond order Number of the electrons in bonding molecule Number of the electrons in the anti-bonding molecule antibonding electrons 2 Furthermore the higher the bond order the more.

. One double bond 2 electron pairs and. 4 rows Bond order can be calculated by the following formula rm Bond order 3. Bond Order Formula Draw the atomic orbitals on the sides. It is defined as half of the difference between them.
Bond order is just several. Most of the time bond order is equal to the number of bonds between two atoms. Usually the higher the bond order the stronger the chemical bond. You add up the total number of bonding pairs and divide by the total number of bonds.
Following the table it can be seen the bond order is 3 because there is a triple bond between the two nitrogen atoms. In the molecular orbital theory bond length bond strength bond order and magnetic properties of different molecules plays an important role. The increase in the value of bond order indicates the strengthening of the bond Formula for. To calculate its bond order using the formula we put 4 for.
For a more complex molecule as The number of bonds is 4 and there is 3. The formula for a bond can be derived by using the following steps. For example for NO 3- you have three bonds. Exceptions occur when the.
For example in diatomic nitrogen NN the bond order is 3. What is the molecular orbital diagram of n2 in this regard. The average bond order formula considers the number of electrons on the bonding and the antibonding orbitals. Bond order is the number of chemical bonds between a pair of atoms and indicates the stability of a bond.
The value zero of the bond order indicates that there is no bond present between the atoms. BO ½ Nb Na Where Nb. Furthermore the Bond Order Formula is frequently defined as half of the difference in the number of electrons in bonding and anti-bonding orbitals. The bond order of a molecule is the average of the bond orders of all the possible structures that describe it.
Bond order is an indication of bond strength. There are 5 valence. It is defined as the difference between the electrons present in the bonding and antibonding orbitals divided by half. The molecular orbitals are in the center brought together by joining the corresponding atomic orbitals.
There is a formula to find the bond order as follows. The bond order of N2 is 25 which is nitrogen ion. Twenty years before the development of quantum mechanics American scientist. We can see from its Lewis structure that the carbon atoms are connected by two bonds which makes four electrons.
The Bond Order Formula can be defined as half of the difference between the number of electrons in bonding orbitals and antibonding orbitals. Bond Order number of bonding electrons number of antibonding electrons 2 Bond Order of F2 8 6 2 22 1 So the bond order. Bond order formula is used to calculate the number of bonds. Bond order number of bonding electrons - number of antibonding electrons 2 Generally the higher the bond order the stronger the bond.
Bond orders of one-half may be stable as shown. Next determine the rate at which coupon. Initially determine the par value of the bond and it is denoted by F.
 |
| Simplest Method To Find Bond Order All Bout Chemistry |
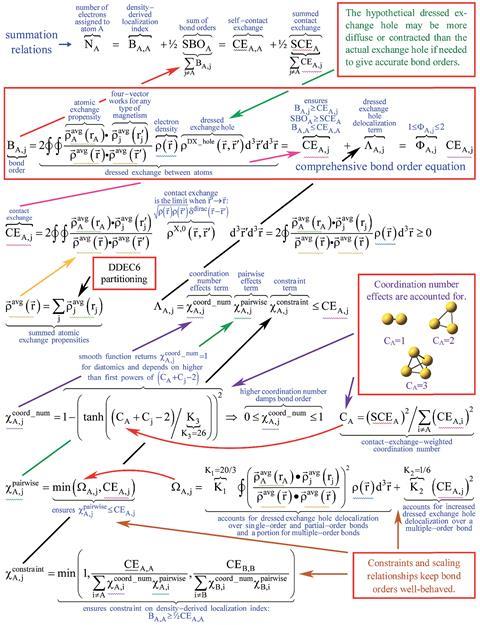 |
| Equation To End Bond Order Contention Research Chemistry World |
 |
| Solved What Is The Bond Order Of S O In So4 2 04 3 3 2 O 2 Chegg Com |
 |
| Trick To Calculate Bond Order Of Polyatomic Ions Youtube |
 |
| Solved Chapter 14 Problem 17e Solution Elements Of Physical Chemistry 6th Edition Chegg Com |
Post a Comment for "bond order formula"