vegetable oil polar or nonpolar
Oil is nonpolar so it can interact with nonpolar molecules like other oil molecules but it cannot interact with polar molecules like water. Hydrophobic substances such as vegetable oil are A nonpolar substances that repel water molecules B nonpolar substances that have an attraction for water molecules C polar substances that repel water molecules D polar substances that have an affinity for water.

Polar And Non Polar Molecules Tmjh 8th Grade Science
This is the reason why the two do not mix well dissolve in one another because they are not soluble in their opposing polarity types.
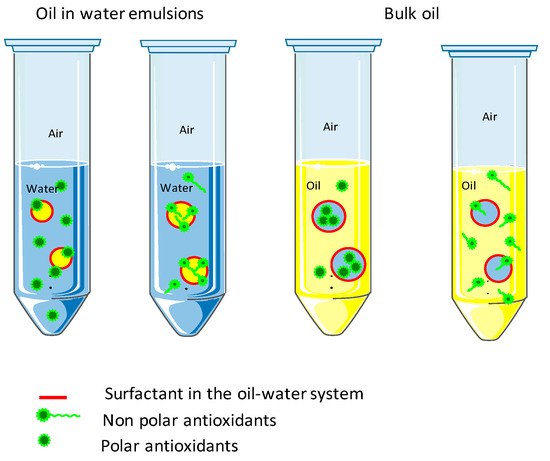

Molecules Free Full Text Vegetable Oils As Alternative Solvents For Green Oleo Extraction Purification And Formulation Of Food And Natural Products Html

What Is Solubility Discovery Express

Molecules Free Full Text Vegetable Oils As Alternative Solvents For Green Oleo Extraction Purification And Formulation Of Food And Natural Products Html


Post a Comment for "vegetable oil polar or nonpolar"